Industry news
How Does Temperature Affect pH
Writer: admin Time:2024-11-05 14:27:45 Browse:3657℃
pH is a crucial parameter in various fields, from chemistry and biology to environmental science and industry. It indicates the acidity or alkalinity of a solution and is vital for processes like fermentation, water treatment, and even human health. However, pH is not a static value; it is influenced by multiple factors, with temperature being one of the most significant. Understanding the interplay between temperature and pH is essential for accurate measurements and effective control of chemical processes.

How Temperature Affects pH Sensors
pH sensors are important tools in both laboratory and industrial settings, providing precise measurements of the pH level in a solution. However, their performance can be significantly affected by temperature changes. At the core of most pH sensors is a glass electrode, which measures the hydrogen ion activity in a solution. The accuracy of this measurement is highly dependent on temperature.
Temperature Compensation: Most pH sensors come equipped with automatic temperature compensation (ATC) features. These sensors adjust the pH reading based on the temperature of the solution, ensuring accuracy. Without ATC, the readings can be skewed, leading to incorrect conclusions and potentially costly errors in processes where pH plays a critical role.
Electrode Response Time: Temperature changes can affect the response time of pH electrodes. At higher temperatures, the response time typically decreases, allowing for quicker adjustments and readings. However, this is not always beneficial, as it can also lead to instability in the measurement if the temperature fluctuates rapidly.
Calibration and Maintenance: Regular calibration of pH sensors is necessary to account for the effects of temperature. Calibration solutions themselves are temperature-sensitive, meaning that calibration should ideally be performed at the same temperature as the sample being measured. Additionally, extreme temperatures can cause physical damage to the electrode, necessitating regular maintenance and replacement.
How to Reduce the Effect of Temperature Changes on pH Measurement
When testing pH, one of the key challenges is dealing with temperature fluctuations that can skew readings. Here’s how you can effectively prevent these errors:
Use a pH Sensor with Automatic Temperature Compensation (ATC):
Choosing a pH sensor that features ATC is your best bet. This technology automatically corrects for temperature effects, ensuring accurate measurements without extra steps.
Manual Temperature Compensation:
If your pH sensor lacks ATC, you’ll need to manually measure the sample's temperature and adjust your calculations accordingly. This step is crucial to achieve precision in your readings.
Avoid Litmus Paper for Accurate Measurements:
Litmus paper lacks the ability to adjust for temperature changes and is therefore not ideal when precise pH readings are required under varying temperatures.
Utilize a Controlled Water Bath:
Placing your buffer solutions and samples in a water bath helps maintain a consistent temperature. By doing so, you minimize the impact of temperature variation, leading to more reliable results.
By following these steps, you can largely eliminate temperature-induced errors in your pH testing, ensuring that your readings are both accurate and consistent.
How Temperature Affects pH
The relationship between temperature and pH is rooted in the fundamental principles of chemistry. Temperature can influence the dissociation of acids and bases in a solution, thereby altering the pH.
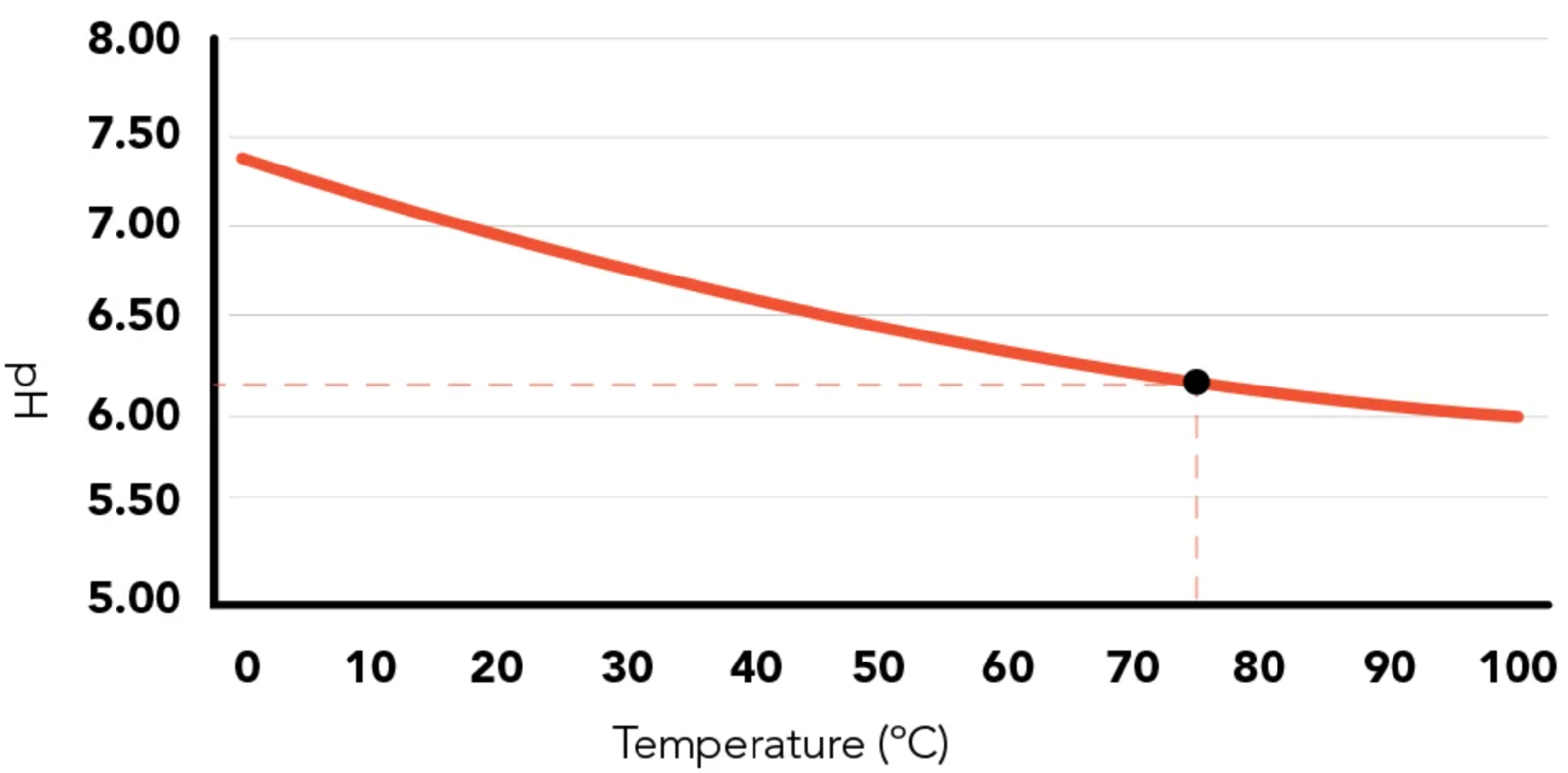
Effect on Water Ionization: Pure water has a pH of 7 at 25°C. However, as temperature increases, the ionization of water also increases, causing the pH to decrease slightly. This means that water at 100°C can have a pH value closer to 6.14, not because it becomes more acidic, but because the neutral point shifts.
Impact on Chemical Equilibria: Many chemical reactions and equilibria are temperature-dependent. For instance, the dissociation of weak acids and bases can be significantly affected by temperature, shifting the pH of the solution. This is particularly important in biological systems, where enzymatic activities are pH-sensitive and can be altered by temperature fluctuations.
Buffer Solutions: Buffers are used to maintain a stable pH in solutions, but their capacity can be compromised by temperature changes. A buffer’s effectiveness is optimal near its pKa value, which can shift with temperature, thus affecting the overall pH stability of the solution.
Practical Implications: In industrial applications, such as fermentation or chemical manufacturing, maintaining an optimal pH is crucial for efficiency and safety. Temperature fluctuations can lead to deviations from the desired pH range, affecting product quality and process stability. Therefore, understanding and mitigating the impact of temperature on pH is essential for process control.
Conclusion
Temperature is a pivotal factor influencing pH and the performance of pH sensors. While technology has provided tools to compensate for temperature effects, awareness and understanding of these influences are crucial for accurate measurements and effective management of chemical processes. Whether in a laboratory, industrial, or environmental setting, considering the temperature’s effect on pH is essential for ensuring the reliability and accuracy of pH readings.
Yosemitech provide online pH sensor for measuring pH. If you have any question or inquiry, feel free to contact us!
Click here to visit our pH sensor product page and learn about more if its features:
https://e.yosemitech.com/pH/Y533-A.html
FAQs
As pH same as acidity?
pH is a measure that indicates how acidic or basic a solution is, on a scale from 0 to 14, with 7 being neutral.
Acidity refers to the concentration of hydrogen ions (H+) in a solution. The more hydrogen ions present, the more acidic the solution is.
Related article:
What does pH value represent?-Yosemite Technologies Co., Ltd_UV254 COD, ODO,pH (yosemitech.com)
Relationship between pH and TDS-Yosemite Technologies Co., Ltd_UV254 COD, ODO,pH (yosemitech.com)
CATEGORIES
CONTACT US
Yosemitech Technologies Co., Ltd
 +86 19984844080
+86 19984844080
 sales@yosemitech.com
sales@yosemitech.com
 Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China
Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China







