Industry news
Understanding Water Hardness: A Comprehensive Guide
Writer: admin Time:2024-08-27 15:24:52 Browse:1714℃
1. What Is Water Hardness?
2. Why We Need to Measure Water Hardness?
3. What Causes Water Hardness?
4. How to Determine Water Hardness from Conductivity or Total Dissolved Soilds Measurements?
5. Is it possible to determine water hardness using conductivity or total dissolved soilds measurements?
6. Conclusion
Water is the basic need for life and its quality has vital impact on our daily life. Hardness is one of the main water quality parameters. Here is a complete guide which talk about, What Water Hardness Is, Why Should Measure It? its Cause, Effects and How to Determine your Conductivity or Total Dissolved Solids Measurements.
1. What Is Water Hardness?
Water hardness is a commonly used indicator of the concentration of divalent cations in water, such as calcium (Ca2+) and magnesium (Mg2+) ions. They dissolve into the water as it slides through rocks and other soil. And how hard is water actually will depend on what and how much of same these minerals are being dissolved in the water.
2.Why We Need to Measure Water Hardness?
Measuring water hardness is important for a variety of reasons, including how it affects our daily life, from housework to personal care to industrial process.
Appliance Efficiency: Hard water can cause scale (mineral deposits) to build in water-heating appliances like kettles, water heaters and boilers. This scale may affect the efficiency of various appliances, resulting in increased energy expenditures and a shorter appliance lifespan. Water hardness measurement allows us to identify the requirement for water softening technologies to safeguard our appliances and save money on electricity.
Personal Care: Hard water can affect skin and hair, causing dryness and irritation. The minerals in hard water can react with soap, forming a residue that can be difficult to rinse off, leading to a "soap scum" effect. This can leave skin feeling itchy and make hair dull and difficult to manage. Understanding water hardness can help individuals adjust their personal care routines and choose appropriate products to mitigate these effects.
Industrial Processes: In industrial settings, hard water can lead to scaling and corrosion in boilers, cooling towers, and other equipment that uses water. This can reduce equipment performance, increase maintenance costs, and lead to potential equipment failure. By measuring water hardness, industries can implement water treatment solutions to prevent these issues, ensuring smooth operation and extending the life of their equipment.
Environmental Considerations: Hard water can also have environmental implications, particularly in areas where water is scarce. Hard water requires more detergent to produce a lather, leading to increased chemical use and potential environmental pollution. By understanding water hardness, we can use appropriate amounts of detergent and adopt more environmentally friendly practices.
Water Treatment and Management: For municipal water suppliers and private well owners, measuring water hardness is crucial for ensuring that the water meets quality standards and is suitable for consumption and other uses. This information is vital for implementing effective water treatment and management
3. What causes water hardness?
Water hardness is mostly determined by the geological composition of the place from which the water is sourced. Groundwater that has come into touch with limestone, chalk, and gypsum is more likely to be hard. As water percolates through these minerals, it dissolves them, resulting in increasing hardness.
Natural versus Man-Made Causes
Water hardness is mostly caused by natural geological formations, although human actions can also play a role. For example, agricultural runoff and industrial waste can bring new minerals into water sources, increasing their hardness.
4. Is it possible to determine water hardness using conductivity or total dissolved soilds measurements?
Yes, water hardness can be estimated from conductivity or total dissolved solids (TDS) measurements, although these methods provide indirect estimates and may not be as accurate as direct hardness testing methods. Here’s how conductivity and TDS can be used to infer water hardness:
1. Conductivity:
Measure Conductivity: Use a conductivity meter to measure the electrical conductivity of the water sample. Conductivity is typically measured in microsiemens per centimeter (µS/cm) or millisiemens per centimeter (mS/cm).
Correlate with Hardness: There is no direct conversion from conductivity to hardness, as conductivity measures all ions, not just calcium and magnesium. However, in areas where calcium and magnesium are the dominant ions, a rough estimate can be made. For example, if the conductivity is known to be primarily due to calcium and magnesium, a general correlation might be applied.
General Correlation: A rough estimate of hardness (in parts per million or milligrams per liter, ppm or mg/L) can be made by dividing the conductivity (in µS/cm) by a factor that accounts for the average contribution of calcium and magnesium to conductivity. This factor can vary but is often in the range of 1.5 to 2.5. For instance:
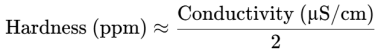
2. Total Dissolved Solids (TDS)
Total Dissolved Solids (TDS) refer to the combined content of all inorganic and organic substances contained in a liquid in molecular, ionized, or micro-granular (colloidal sol) suspended form. TDS is often used as an indicator of water quality and can be correlated with water hardness.
Measure TDS: Use a TDS meter to measure the total dissolved solids in the water sample. TDS is typically measured in parts per million (ppm) or milligrams per liter (mg/L).
Correlate with Hardness: Similar to conductivity, there is no direct conversion from TDS to hardness. However, in areas where TDS is primarily due to calcium and magnesium, a correlation can be established. For example, if TDS is known to be largely composed of calcium and magnesium, a general correlation might be applied.
General Correlation: A rough estimate of hardness can be made by assuming that a certain percentage of the TDS is due to calcium and magnesium. This percentage can vary but is often in the range of 50% to 80%. For instance:
![]()
Limitations and Considerations
Accuracy: Both conductivity and TDS provide indirect estimates of water hardness. The accuracy of these estimates depends on the specific composition of the water and the dominance of calcium and magnesium ions.
Local Conditions: The correlations used to estimate hardness from conductivity or TDS can vary based on local geological conditions and the presence of other ions.
Direct Testing: For more accurate measurements, direct hardness testing methods such as titration or using water hardness test kits are recommended. These methods specifically measure the concentration of calcium and magnesium ions.
In summary, while conductivity and TDS measurements can provide a rough estimate of water hardness, they are not as precise as direct hardness testing methods. These estimates are most useful in situations where a quick approximation is needed, and local conditions allow for a reasonable correlation between conductivity/TDS and hardness.
CATEGORIES
CONTACT US
Yosemitech Technologies Co., Ltd
 +86 19984844080
+86 19984844080
 sales@yosemitech.com
sales@yosemitech.com
 Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China
Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China







