Industry news
How to Select Dissolved Oxygen Sensor (Optical, Galvanic, Polarographic)?
Writer: admin Time:2024-08-07 13:30:01 Browse:1761℃
1. Dissolved Oxygen and Water Quality
Oxygen is an important component of air and is essential for human breathing and the growth of plants and animals. Oxygen flows through the air and is dissolved in water by plant photosynthesis. This part of oxygen dissolved in water is called dissolved oxygen. Dissolved oxygen in water is mainly supplemented by air and photosynthesis, but it is also consumed by the respiration and decomposition of plants and animals in water.
Dissolved oxygen is one of the important indicators of water quality monitoring and a key factor in measuring the self-purification capacity of water bodies. Ideally, the dissolved oxygen content of clean water bodies is close to saturation. In water, if there are a large number of algae plants, the oxygen released by their photosynthesis can make the dissolved oxygen in the water reach a supersaturated state. However, when the water body is polluted by organic matter or a large number of algae die, the dissolved oxygen will continue to be consumed and reduced, and even cause the water body to enter an anaerobic state. In this case, anaerobic microorganisms will multiply, and organic matter will decompose and cause the water body to appear black and smelly.

2. Different Methods to Measure Dissolved Oxygen
Generally, dissolved oxygen analyzers are mainly used to measure the oxygen content in water. Common types of dissolved oxygen sensors include: Optical, Galvanic, Polarographic DO Sensor
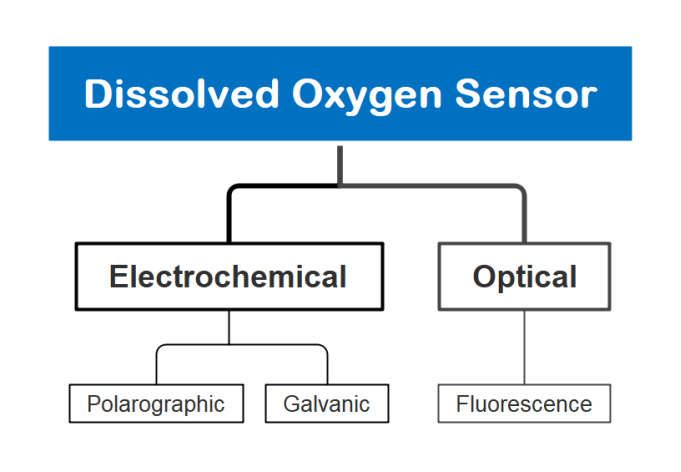
2.1 Electrochemical Dissolved Oxygen Sensor Working Principle
Both galvanic and polarographic dissolved oxygen (DO) sensors are types of electrochemical sensors used for measuring dissolved oxygen levels.
In an electrochemical dissolved oxygen sensor, dissolved oxygen diffuses from the sample through an oxygen permeable membrane into the sensor. Once in the sensor, the oxygen undergoes a chemical reduction reaction, generating an electrical signal. This signal can be read by a dissolved oxygen instrument.
2.2 Polarographic vs. Galvanic DO Sensors
Polarographic dissolved oxygen sensors use electrochemical polarography to measure oxygen concentration by generating current changes in the reduction reaction of dissolved oxygen. The sensor usually includes a working electrode, a reference electrode, and a counter electrode. Its advantages are high sensitivity and low detection limit, which are suitable for applications in scientific research and high-precision measurement. However, due to its sensitivity to temperature and pressure changes, stable environmental conditions must be maintained when used.
Amperometric dissolved oxygen sensors measure by detecting the current generated by the electrochemical reaction of oxygen on the electrode surface. Compared with polarography, this sensor has a faster response speed and is usually not affected by temperature, making it suitable for online monitoring and real-time data acquisition. However, its sensitivity and accuracy are relatively low, making it suitable for general industrial applications that are not sensitive to changes in oxygen concentration.
Therefore, polarography is suitable for high-precision scientific research, and the galvanic method is more suitable for real-time monitoring.
2.3 Optical Dissolved Oxygen Sensor Working Principle
The fluorescence dissolved oxygen meter is based on the principle of fluorescence quenching. The instrument excites the fluorescent substance to emit red light by irradiating it with blue light. Oxygen molecules will take away part of the excitation energy through the quenching effect, so the time and intensity of the red light excitation are inversely proportional to the oxygen concentration. By measuring the phase difference between the excitation red light and the reference light and comparing it with the internal calibration value, the concentration of oxygen molecules can be accurately calculated. After linearization and temperature compensation, the instrument finally outputs an accurate dissolved oxygen concentration value.
2.4 Optical vs. Galvanic DO Sensors
Optical dissolved oxygen sensors use fluorescence technology to measure the concentration of dissolved oxygen by exciting specific fluorescent materials. Under the action of light, the luminescence intensity of the fluorescent material changes with the concentration of dissolved oxygen. The advantages of this sensor include no oxygen consumption, no flow rate restriction, fast response speed, high stability, strong anti-interference, and low maintenance cost. Optical sensors also have good durability and are suitable for long-term monitoring.

https://e.yosemitech.com/DO/Y504-A.html
Electrochemical dissolved oxygen sensors measure oxygen concentration based on changes in current. They usually consist of an anode and a cathode, and generate current signals through electrochemical reactions. Although their cost is relatively low, they require regular calibration and maintenance during use and are sensitive to changes in temperature and pressure. In addition, the performance of electrochemical sensors may be affected in highly polluted environments.
In summary, optical dissolved oxygen sensors are more suitable for applications that require long-term continuous monitoring, while electrochemical dissolved oxygen sensors may have an advantage in cost-sensitive projects. Choosing the right sensor requires comprehensive consideration based on specific application requirements, environmental conditions, and budget.
3. Dissolved Oxygen Sensor Application

Municipal and industrial wastewater treatment, including regulating tanks, aeration tanks, aerobic and anaerobic digestion tanks, and effluent testing.

Water environment monitoring of rivers, lakes, seawater, fisheries, etc.
CATEGORIES
CONTACT US
Yosemitech Technologies Co., Ltd
 +86 19984844080
+86 19984844080
 sales@yosemitech.com
sales@yosemitech.com
 Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China
Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China







