Industry news
What does pH value represent?
Writer: admin Time:2024-05-27 15:33:01 Browse:1398℃
“pH” is one of the most commonly used water quality monitoring indicators. The pH value measures the concentration of hydrogen ions in water-based liquids, indicating the acidity or alkalinity of the substance. But what exactly does pH represent, and why is it so important to us? This comprehensive guide will delve into the concept of pH, its measurement, the factors that influence it, its applications across various fields, and the importance of maintaining proper pH levels.
What Is pH?
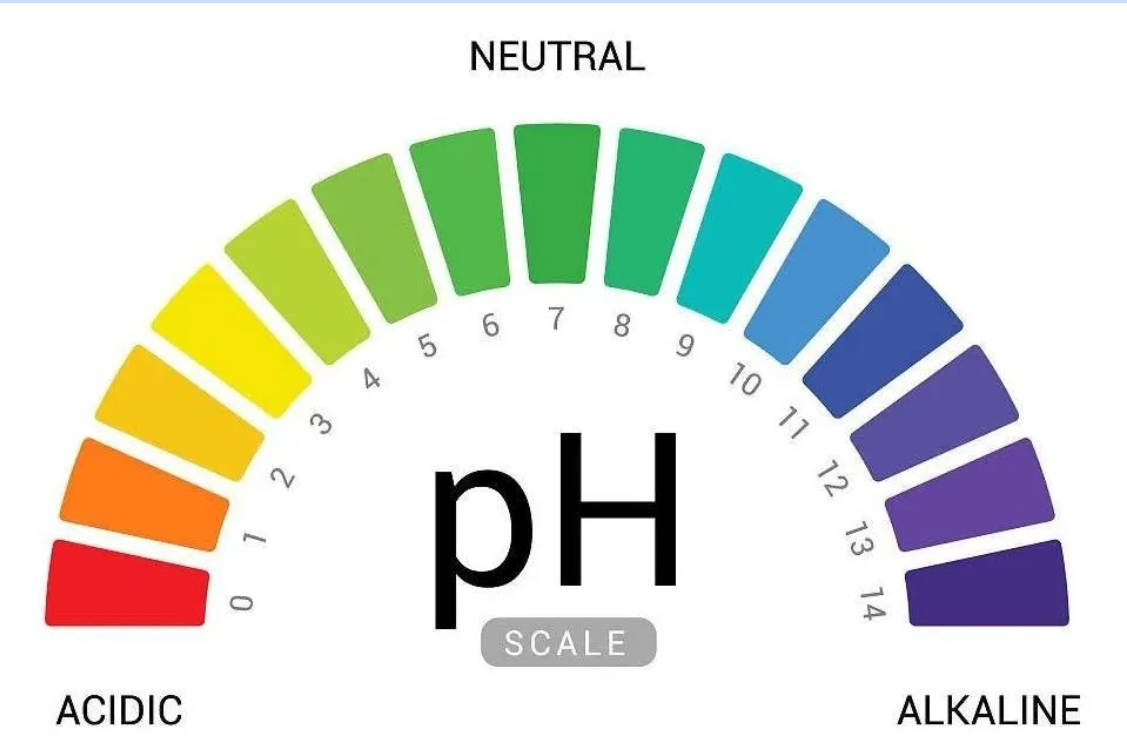
The pH scale measures the acidity or basicity of a solution by indicating the concentration of hydrogen ions in a liquid. A low pH indicates a higher concentration of hydrogen ions, while a high pH indicates a lower concentration. The pH scale is a number scale from 0 to 14. Neutral solutions are exactly pH 7. Acidic solutions have pH values less than 7. The closer to pH 0, the more acidic a solution is. Alkaline solutions have pH values more than 7. The closer to pH 14, the more alkaline a solution is.
Why pH Value Is So Important to Us?
The importance of pH in our lives cannot be overstated. It plays a crucial role in various biological processes, chemical reactions, and environmental conditions. For instance, the pH of our blood must be maintained within a narrow range for us to survive. Similarly, the pH of soil affects plant growth, the pH of water impacts aquatic life, and the pH of food influences its taste and safety.
Measuring pH
Methods of pH Measurement
pH meters are electronic devices used to measure the pH of a solution. They work by measuring the voltage difference between two electrodes immersed in the solution. The voltage is then converted into a pH reading. pH meters are accurate and widely used in laboratories, industries, and fieldwork.

(2) pH Indicator Papers and Strips
pH indicator papers and strips are coated with pH-sensitive dyes that change color in response to different pH levels. They are easy to use and provide a quick, albeit less precise, measure of pH. These strips are often used for preliminary tests or in educational settings.

(3) pH Indicator Solutions
pH indicator solutions contain dyes that change color at different pH levels. When added to a solution, the color change indicates the pH range of the solution. Common indicators include litmus, phenolphthalein, and methyl orange.
Calibration and Accuracy of pH Measurements
For accurate pH measurements, it's essential to calibrate pH meters regularly using standard buffer solutions of known pH. Calibration ensures that the meter provides precise readings, which are crucial for scientific research, quality control, and medical diagnostics.
Factors Influencing pH
Concentration of Hydrogen Ions
The concentration of hydrogen ions (H+) directly determines the pH of a solution. An increase in H+ concentration lowers the pH (making the solution more acidic), while a decrease raises the pH (making the solution more alkaline).
Temperature Effects
Temperature can affect pH readings because it influences the activity of hydrogen ions in a solution. Most pH meters have a temperature compensation feature to adjust for this effect, ensuring accurate readings across different temperatures.
Presence of Other Ions and Compounds
The presence of other ions and compounds can also influence pH. For example, the presence of strong acids or bases can significantly alter the pH of a solution. Additionally, certain salts can hydrolyze in water, changing the pH.
Applications of pH
Agriculture and Soil Science
In agriculture, the pH of soil is crucial for plant growth. Different plants thrive in different pH ranges. Farmers use pH measurements to adjust soil acidity or alkalinity to optimize crop yields.
Aquatic Environments and Water Quality
The pH of water bodies is vital for aquatic life. Acidic or alkaline conditions can harm fish and other aquatic organisms. Monitoring water pH helps in managing water quality and protecting ecosystems.
Medicine and Healthcare
In medicine, pH is important for diagnosing and treating various conditions. For example, the pH of blood must be maintained within a narrow range (7.35-7.45) for proper bodily functions. Deviations from this range can indicate serious health issues.
Food and Beverage Industry
The pH of food and beverages affects their taste, texture, and safety. Acidity can inhibit the growth of harmful bacteria, making acidic foods more shelf-stable. Additionally, the pH of ingredients can affect the outcome of cooking and baking processes.
Industrial Processes
In industries, pH control is essential for various processes, such as water treatment, chemical manufacturing, and metal processing. Accurate pH measurements ensure product quality and safety, as well as environmental compliance.
Importance of Maintaining Proper pH Levels
Biological Systems and Homeostasis
Maintaining proper pH levels is critical for the survival of living organisms. Biological systems, such as the human body, have mechanisms to regulate pH and maintain homeostasis. Disruptions in pH can lead to disease and death.
Chemical Reactions and Processes
The rate and outcome of chemical reactions are often pH-dependent. Controlling pH is essential for achieving desired results in chemical synthesis, industrial processes, and biological systems.
Environmental Impact
Environmental conditions, such as acid rain and ocean acidification, can have devastating effects on ecosystems. Monitoring and managing pH levels are crucial for protecting the environment and preserving biodiversity.
Conclusion
In conclusion, the pH value serves as a fundamental indicator of chemical properties in diverse realms, shaping our understanding of acidity and alkalinity. With the aid of precise instruments like pH meters, researchers, scientists, and industry professionals can measure and monitor pH levels with accuracy and efficiency. By grasping the essence of pH value and its implications, we can navigate the complexities of our surroundings with greater insight and clarity.
Related articles:
https://e.yosemitech.com/industry/102.html
https://e.yosemitech.com/industry/What-pH-standard-should-drinking-water-be.html
https://e.yosemitech.com/industry/What-pH-standard-should-drinking-water-be.html
CATEGORIES
CONTACT US
Yosemitech Technologies Co., Ltd
 +86 19984844080
+86 19984844080
 sales@yosemitech.com
sales@yosemitech.com
 Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China
Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China







