Industry news
How to Test and Adjust the pH of Your Water Source: A Complete Guide for Safe Water
Writer: admin Time:2025-03-14 09:47:54 Browse:1920℃
Water pH is a critical indicator of water quality, affecting everything from human health to aquatic ecosystems. Whether you're managing a household water supply, maintaining a fish tank, or ensuring safe drinking water, understanding how to test and adjust pH levels is essential. This comprehensive guide will walk you through the science, tools, and practical steps to achieve balanced water pH for safety and sustainability .
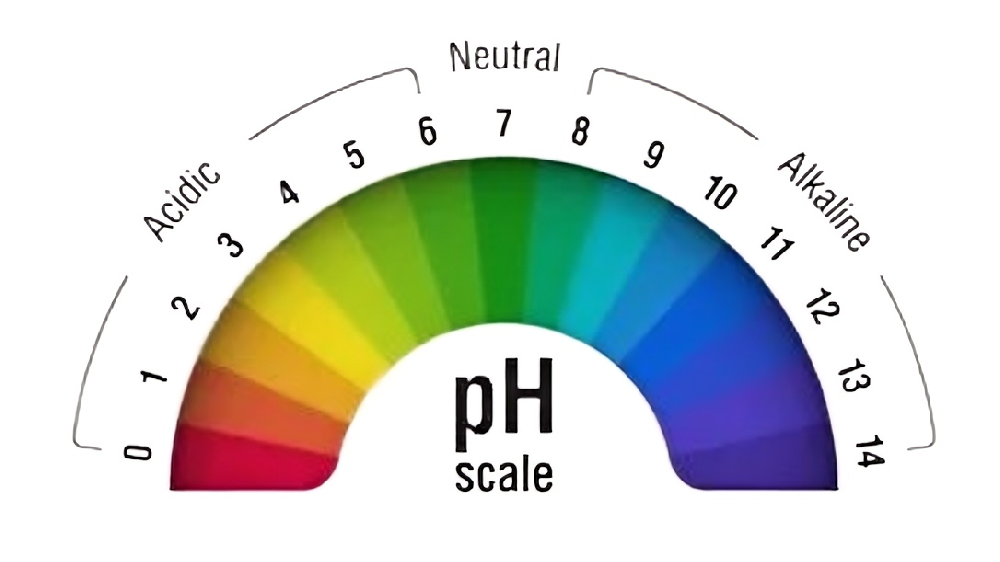
1. Why pH Matters for Water Safety
1.1 The Basics of pH
pH measures the acidity or alkalinity of water on a scale of 0–14, with 7 being neutral. Values below 7 indicate acidity (e.g., lemon juice), while values above 7 signify alkalinity (e.g., baking soda). Even slight deviations from the ideal range can impact water safety:
Health Risks: Acidic water (pH < 6.5) may corrode pipes, leaching heavy metals like lead into drinking water. Alkaline water (pH > 8.5) can cause gastrointestinal discomfort and reduce chlorine’s disinfection efficacy .
Environmental Impact: Aquatic life, especially fish, is highly sensitive to pH changes. For example, koi thrive in pH 7.5–8.2, while deviations can lead to stress or death .
1.2 Ideal pH Ranges for Different Water Sources
Drinking Water: 6.5–8.5 (WHO standards) .
Aquariums: 6.5–7.5 for tropical fish; saltwater tanks may require 8.0–8.4 .
Gardening: Most plants prefer slightly acidic water (pH 5.5–6.5) .
Swimming Pools: 7.2–7.8 to prevent eye irritation and equipment corrosion.
2. How to Test Water pH Accurately
2.1 Tools for pH Testing
Choose the right method based on your needs:
pH Test Strips
Pros: Affordable, easy to use, and portable.
Cons: Less precise (±0.5 pH units). Ideal for quick checks in home aquariums or pools .
Steps: Dip the strip into water, wait 10 seconds, and compare the color to a reference chart.
pH Sensors/Meters
Pros: High accuracy (±0.01 pH units). Suitable for labs, farms, or industrial use.
Cons: Requires calibration and maintenance.
Steps:Calibrate with buffer solutions (pH 4.0, 7.0, and 10.0).
Immerse the electrode in water, wait for stabilization, and record the reading .
Liquid Test Kits
Pros: More accurate than strips. Used for detailed analysis in gardening or drinking water.
Cons: Time-consuming.
Steps: Add reagent drops to a water sample, shake, and compare the color change .
2.3 Common Mistakes to Avoid
Contaminated Samples: Always use clean containers to avoid skewed results.
Temperature Effects: pH meters must compensate for temperature (e.g., 25°C is standard) .
Expired Reagents: Check expiration dates on test strips or liquid kits.
3. Adjusting Water pH: Step-by-Step Solutions
3.1 Raising pH (Reducing Acidity)
For Drinking Water:
Add pH drops or install a neutralizing filter with calcite .
For Aquariums:
Use crushed coral or baking soda (1 tsp per 5 gallons). Monitor changes gradually to avoid shocking fish .
For Pools:
Add sodium carbonate (soda ash). For a 10,000-gallon pool, 6 oz raises pH by 0.2 .
3.2 Lowering pH (Reducing Alkalinity)
For Drinking Water:
Install an acid injection system or add lemon juice (natural citric acid) .
For Gardens:
Use peat moss or elemental sulfur to acidify soil irrigation water .
For Aquariums:
Add driftwood or almond leaves, which release tannins .
4. Specialized Scenarios
4.1 Testing and Adjusting High-Purity Water
Ultra-pure water (e.g., lab-grade) lacks ions, making pH measurement unstable. Use specialized electrodes with low ionic strength compensation.
4.2 Managing pH in Large-Scale Systems
Municipal Water Treatment: Lime (calcium hydroxide) is added to raise pH, while CO2 injection lowers it.
Industrial Boilers: Maintain pH 8.5–9.5 to prevent corrosion. Automated sensors and dosing pumps ensure consistency.
5. Maintaining Balanced pH Long-Term
Regular Monitoring: Test weekly for aquariums/pools and annually for household water.
Preventive Measures:
Use corrosion-resistant pipes (e.g., PVC) to avoid metal leaching.
Install whole-house filters with pH-balancing cartridges.
Documentation: Keep logs to track trends and adjust treatments proactively.
FAQs
1. How often should I test the pH of my water source?
It depends on the application. For drinking water, testing every few months is recommended. For aquariums and hydroponics, weekly testing is ideal.
2. Can I use household items to test pH levels?
While some household items like red cabbage juice can give a rough estimate of pH, they are not as accurate as pH test strips, liquid test kits, or digital pH meters.
3. What is the ideal pH range for drinking water?
The ideal pH range for drinking water is between 6.5 and 8.5.
4. How do I know if my pH meter needs calibration?
If your pH meter consistently gives readings that are off from expected values, it may need calibration. Regular calibration is recommended to ensure accuracy.
5. Can pH levels affect the taste of my water?
Yes, water with a pH outside the optimal range can taste metallic, bitter, or sour.
6. What are the signs of pH imbalance in an aquarium?
Signs include fish stress, algae blooms, and changes in water clarity.
7. How do I adjust the pH in my garden's water source?
Use pH decreasers or increasers as needed, and regularly test the pH of your irrigation water.
8. Are there any natural ways to adjust pH levels?
Yes, natural methods include using peat moss, vinegar, limestone, and baking soda.
9. What are the risks of using chemicals to adjust pH levels?
Overuse or improper handling of chemicals can lead to health risks and environmental damage. Always follow safety guidelines.
10. How can I maintain stable pH levels in my aquarium?
Regularly test the water, use appropriate pH adjusters, and ensure proper filtration and aeration.
Related Articles:
https://e.yosemitech.com/industry/What-pH-standard-should-drinking-water-be.html
https://e.yosemitech.com/industry/How-Does-a-pH-Probe-Work.html
https://e.yosemitech.com/industry/Does-Chlorine-Increase-or-Decrease-Pool-pH.html
https://e.yosemitech.com/industry/Digital-vs-Analog-pH-Meters.html
https://e.yosemitech.com/industry/How-Does-Temperature-Affect-pH.html
https://e.yosemitech.com/industry/How-to-Safely-Raise-pH-in-Your-Aquarium.html
CATEGORIES
CONTACT US
Yosemitech Technologies Co., Ltd
 +86 19984844080
+86 19984844080
 sales@yosemitech.com
sales@yosemitech.com
 Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China
Bldg,25,CECEP Industrial Park, No. 18 Dongchang Rd. Suzhou Industrial Park, Jiangsu Province,China 215126, China







